What is Myriad myRisk® Hereditary Cancer?
Myriad myRisk® Hereditary Cancer panel represents the next generation of hereditary cancer risk testing, blending both genetic test status and personal cancer family history.
Myriad myRisk® Hereditary Cancer is a comprehensive solution designed to help both patients and specialists learn about factors that determine and manage personal hereditary risks of many types of cancer, based on a multi-gene panel involved in the 8 most frequently occurring hereditary based cancers.
Each gene tested with Myriad myRisk links to one or more of most frequent hereditary cancer sites, as Breast Cancer, Colorectal Cancer, Melanoma, Gastric Cancer, Ovarian Cancer, Endometrial Cancer, Pancreatic Cancer, Prostate Cancer and rare types of cancer.
Myriad myRisk simplifies the test selection process by providing the most comprehensive hereditary cancer panel test with clinically significant results. Additionally, Myriad myRisk provides a summary of professional medical guidelines to help healthcare specialists optimize their patients’ medical management.
Who should take the test?
Myriad myRisk® Hereditary Cancer is appropriate for individuals with either a personal or a family (close relatives: parent, sibling, child, uncle, aunt, great uncle, great aunt, nephew, niece, grandparent, grandchild, or half-sibling) history of any one of the following cancer pathology: Breast Cancer, Colorectal Cancer, Melanoma, Gastric Cancer, Ovarian Cancer, Endometrial Cancer, Pancreatic Cancer, Prostate Cancer, Other*, that are in one of the situations below:
![]() Mininum 2 types of above listed cancers that affect or have affected the individual and/or one or more of the close relatives, or
Mininum 2 types of above listed cancers that affect or have affected the individual and/or one or more of the close relatives, or
![]() Cancer occuring at young age (50 years or younger) that affect or have affected the individual and/or one or more of the close relatives or
Cancer occuring at young age (50 years or younger) that affect or have affected the individual and/or one or more of the close relatives or
![]() A rare type of cancer that affects or have affected the individual and/or one or more of the close relatives at any age, with any of the following features: male breast cancer, triple negative breast cancer, ovarian cancer, abnormal microsatellite instability (MSI)/immunohistochemistry (ICH) or abnormal histology.
A rare type of cancer that affects or have affected the individual and/or one or more of the close relatives at any age, with any of the following features: male breast cancer, triple negative breast cancer, ovarian cancer, abnormal microsatellite instability (MSI)/immunohistochemistry (ICH) or abnormal histology.
Capturing a thorough family history will allow healthcare specialists to better evaluate their patients’ hereditary cancer risk and appropriateness for testing.
*Other Lynch syndrome-associated cancers, 10 or more gastrointestinal adenomatous polyps.
What are the Clinical Benefits of the test?
Using an extensive number of sophisticated technologies and proprietary algorithms, unsurpassed lab accuracy, and industry leading variant classification, Myriad myRisk offers physicians important advantages for the most informed medical decisions possible.
The test revolutionizes hereditary cancer testing and patient care, as it:
![]() Captures more mutation carriers than the analysis of BRCA1 and BRCA2 for susceptibility to Hereditary Breast and Ovarian Cancer syndrome and Lynch syndrome (Hereditary Colorectal Cancer and Uterine Cancer) combined;
Captures more mutation carriers than the analysis of BRCA1 and BRCA2 for susceptibility to Hereditary Breast and Ovarian Cancer syndrome and Lynch syndrome (Hereditary Colorectal Cancer and Uterine Cancer) combined;
![]() Blends genetic testing status and personal/family cancer history into clinically actionable risk assessment and follow-up;
Blends genetic testing status and personal/family cancer history into clinically actionable risk assessment and follow-up;
![]() Provides specific medical management recommendations for patients who test positive or negative based on the guidelines of leading professional medical societies;
Provides specific medical management recommendations for patients who test positive or negative based on the guidelines of leading professional medical societies;
![]() Provides a test report that is simple, clear and easy to use.
Provides a test report that is simple, clear and easy to use.
Myriad myRisk also includes riskScore® for certain patients, a groundbreaking breast cancer risk-prediction tool.
![]() riskScore is a precision medicine tool that predicts a woman’s five year and lifetime risk for developing breast cancer.
riskScore is a precision medicine tool that predicts a woman’s five year and lifetime risk for developing breast cancer.
![]() riskScore analyzes over 80 genetic markers combined with the Tyrer-Cuzick model to estimate a woman’s risk for developing breast cancer.
riskScore analyzes over 80 genetic markers combined with the Tyrer-Cuzick model to estimate a woman’s risk for developing breast cancer.
![]() riskScore is calculated for women under age 85, of solely European ancestry, without a personal history of breast cancer, LCIS, hyperplasia, atypical hyperplasia, or a breast biopsy with unknown results.
riskScore is calculated for women under age 85, of solely European ancestry, without a personal history of breast cancer, LCIS, hyperplasia, atypical hyperplasia, or a breast biopsy with unknown results.
What DNA sample is required to perform the test?
Myriad myRisk® Hereditary Cancer test requires simple saliva sample that can be easily and painlessly collected within seconds by any qualified healthcare professional.
Frequently Asked Questions
What criteria were used for the selection of genes in the Myriad myRisk panel?
Genes were selected by review of medical societal guidelines, cumulative studies, and discussion with experts in cancer genetics. Cancer risks in these genes have a minimum of 2-3 fold increase over the general population. Attention was focused on existing well defined cancer genetics syndromes, genes for which their contribution to adult cancer is well understood and newly discovered genes associated with inherited cancer.
The genes analyzed with Myriad myRisk are APC, ATM, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A (p16INK4a and p14ARF), CHEK2, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD51C, RAD51D, SMAD4, STK11, TP53.
How do you know the Myriad myRisk NGS platform is as sensitive as BRACAnalysis®?
In a BRCA1 and BRCA2 comparison study NGS identified 15,877 sequence variants while Sanger sequencing identified 15,878. Based on these results, the NGS process was refined prior for the validation of the full gene panel.
In the validation study, NGS and Sanger sequencing were 100% concordant for the 3,923 collective variants across all genes for an analytical sensitivity of the NGS assay of >99.92% and analytical specificity >99.99% (lower limit of 95% confidence interval).
Myriad myRisk is shown in additional validation to be highly effective and provided accurate results equivalent to those obtained from Sanger DNA sequencing analysis.
What is the turnaround time for Myriad myRisk Test Report?
The majority of myRisk results are completed within 21 days. We will notify your prescribing physician in the unusual event that results are taking longer than 21 days.
What are the references for the Myriad myRisk Test Report medical management recommendations?
The myRisk Management Tool reference the National Comprehensive Cancer Network (NCCN), International Cancer of Pancreas Screenings (CAPS), Claus breast cancer risk model, Amsterdam Criteria, and others to provide medical management considerations.
For example, patients with family histories and/or a gene mutation that is associated with >20% lifetime risk for breast cancer will be presented with NCCN based consideration of improved breast screening.
How does the test work?
Myriad used our experience with over a million patients tested to further optimize Myriad myRisk® testing with next generation sequencing (NGS). NGS allows for rapid sequencing of multiple genes concurrently.
In the largest validation study of its kind, Myriad estimated analytical sensitivity of greater than 99.92% with 100% concordance on NGS/Sanger parallel sequencing in all genes in the Myriad myRisk panel and 100% concordance on large rearrangement validation.
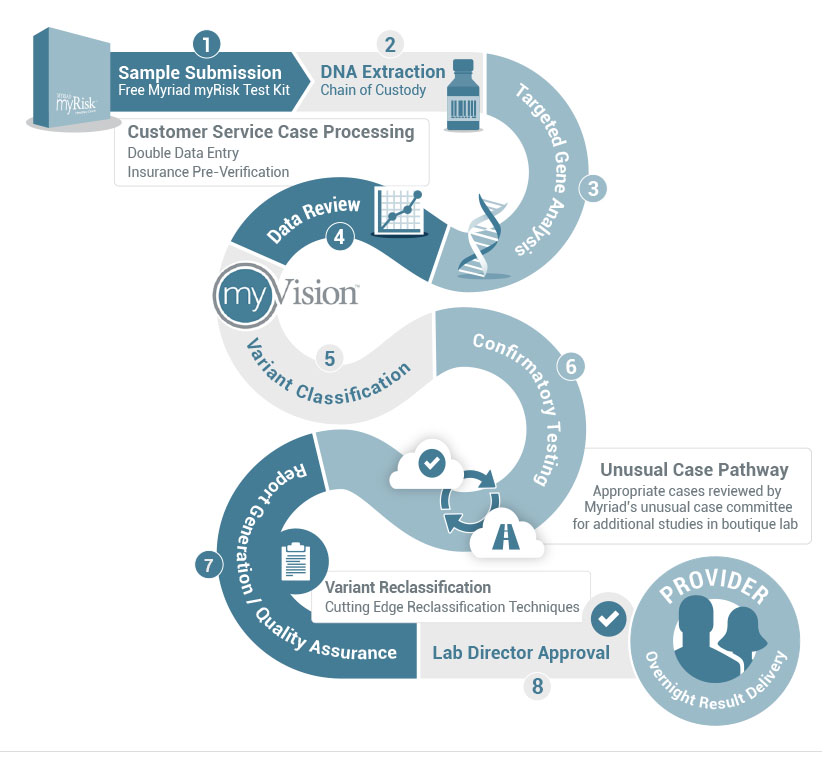
Myriad myRisk’s gold standard NGS technology serves as only one part of the many steps that Myriad pursues to ensure the highest quality and sensitivity for every sample that is submitted. The following cycle shows the extensive laboratory process from DNA extraction and sequencing to laboratory director approved reports.
Why Variants of Uncertain Significance (VUS) are extremely important?
Myriad is the unquestioned industry leader in variant classification and reclassification. myVision™ is the most advanced program in the industry overseeing the classification and reclassification of uncertain variants and is part of Myriad’s commitment to patients and their families that lasts a lifetime.
Variation in human genes is enormous, with new genetic changes discovered every day. That’s why variants of uncertain significance (VUS) are expected in genetic testing. For a patient who undergoes genetic testing, a VUS result can be confusing; for healthcare providers, counseling these patients can be a challenge:
![]() A VUS result raises questions yet offers no clear answers for patients, families, or healthcare providers
A VUS result raises questions yet offers no clear answers for patients, families, or healthcare providers
![]() Positive results trigger cancer risk management plans
Positive results trigger cancer risk management plans
![]() Negative results help avoid unnecessary screenings and surgery
Negative results help avoid unnecessary screenings and surgery
Myriad’s leadership in hereditary cancer testing
Over the past 20 years, Myriad has conducted over a million genetic tests for hereditary cancer. The resulting analysis and interpretation of the variant database support the Myriad commitment to reduce VUS in genetic test results and ensure:
![]() Accurate, clinically significant results from every Myriad test performed
Accurate, clinically significant results from every Myriad test performed
![]() Reduced uncertainty for patients and families
Reduced uncertainty for patients and families
![]() Confidence for providers counseling their patients
Confidence for providers counseling their patients
Learn more about the VUS Family Testing program for Myriad myRisk Hereditary Cancer Panel testing.
The myRisk test report provides clear and actionable direction.
One of the main advantages of Myriad myRisk is represented by the test result that doctors and patients receive, in a clear and easy to use report that stands as a valuable clinical decision support tool.
Every report includes:
![]() myRisk Genetic Result, providing the diagnosis and information on any clinically significant mutations identified;
myRisk Genetic Result, providing the diagnosis and information on any clinically significant mutations identified;
![]() guideline based medical management considerations for both positive and negative results;
guideline based medical management considerations for both positive and negative results;
![]() family history analysis based on increased risk logic of guidelines;
family history analysis based on increased risk logic of guidelines;
![]() information for family members.
information for family members.

Subscribe for a test
If you or someone you know might be interested in benefiting of Myriad myRisk® science to help you choose the best treatment option, subscribe here and one of our experts will setup a planning for you to gain access to this genetic test. Tell us more about your interest and inquiries by filling in the next contact form and we’ll be in touch shortly.

Schedule for a consultation
The testing process may be complex and it’s essential to have a trusted resource when making decisions. With the help of our partner physicians, experts in genetic counseling in hereditary cancer risk testing, you will find out the benefits of the Myriad myRisk® test, understand the clinical results and make the right decisions for your future health. Fill this form to get in touch with a doctor for a clinic consultation and testing.
